In Vitro Intestinal Toxicity Testing
HemoGenix® is a Compliant Contract Research Service Supplier through Scientist.com
The cells of the intestine represent one of the five primary, continuously proliferating cells systems of the body, the other being the lympho-hematopoietic system, the corneal cells of the eye, cells of the reproductive system and the skin. All five systems demonstrate the same stem cell heiarachy as epitomized by the lympho-hematopoietic system. However, unlike the lympho-hematopoietic system, the assays presently available to understand the response of cells to perturbations are not as well developed. Nevertheless, with the availability of primary human intestinal cell populations, in vitro toxicity testing can be performed using the HemoGenix® EpiGlo™-Tox HT Platform.
For more information on in vitro intestinal toxicity testing, please contact HemoGenix® at contractresearch@hemogenix.com or call (719) 264-6250.
Types of Toxicity Studies Available
The majority of studies performed by HemoGenix® incorporate a Complete Service, Full Report that can be used for an IND application. More recently, Sponsors have requested a more streamlined study report primarily for screening purposes. The Rapid Tox Study Report is a fast and cost-effective alternative.
- Complete Service, Full Report Study: Fully customized study that includes the Study Plan, Draft Text and Final Text Report with QA audit.
- Rapid Toxicity Study Report: Perfect for high-throughput screening during early drug development and for studies that do not require a formal report. A customized study that includes the Study Plan and full protocol, raw results and graphical data in a single Excel Workbook. No formal text report and no QA audit is performed. No text report, discussion, interpretation or conclusions are provided. This type of report can also be used with our ComparaTOX™ Platform.
With the introduction of standardized and validated ATP bioluminescence in vitro toxicity assays starting with HALO®-Tox HT for bone marrow toxicity/stem cell hematotoxicity testing in 2002, HemoGenix® has developed a number of in vitro toxicity testing assay platforms. EpiGlo™-Tox HT for in vitro intestinal toxicity testing joins the family of ATP bioluminescence toxicity testing assays that also include HepatoGlo™-Tox HT, CardioGlo™-Tox HT, RenalGlo™-Tox HT and SkinGlo™-Tox HT. All of these can be performed in parallel with each other, since they all use the same readout. This extended toxicity testing platform is called the ComparaTox™ Platform.
EpiGlo™-Tox HT for In Vitro Drug-Induced Intestinal Toxicity Testing
- Many primary intestinal cell populations, e.g. epithelial cells and myofibroblasts, exhibit proliferation that correlates with the intracellular ATP concentration measured using EpiGlo™-Tox HT .
- EpiGlo™Tox HT can be used for intestinal cells from various species..
- EpiGlo™-Tox HT incorporates a validated ATP bioluminescence readout to measure intestinal toxicity.
- EpiGlo™-Tox HT is a calibrated and standardized assay platform that allows results to be directly compared between different drugs, as well as different cell types over time.
- EpiGlo™-Tox HT is a high-throughput assay platform using 96- or 384-well plate formats allowing ADME-Tox drug or compound screening, thereby significantly reducing unexpected results during pre-clinical testing.
- EpiGlo™-Tox HT can be used to investigate cellular drug-drug interactions or multiplexed with assay readouts.
- Incorporates the most sensitive ATP bioluminescence readout available.
- Study Turnaround time: Usually within 4-5 days.
- Validated assay readout according to FDA Bioanalytical Method Guidelines.
- Supports the 3Rs (Reduction, Refinement, Replacement) assay platform for animal testing.
Intestinal Cell Sources for EpiGlo™-Tox HT
- Cryopreserved
- Cell lines of the intestinal tract
Species Available for Use With EpiGlo™-Tox HT
EpiGlo™-Tox HT can be used with proliferating intestinal cells from a variety of animal species.
Please contact HemoGenix® for more information.
Multiplexing Capabilities with EpiGlo™-Tox HT
- Membrane integrity: LDH or PI dye exclusion
- Apoptosis: Biochemical caspase detection
- Mitochondrial dysfunction: Mitochondrial ToxGlo™
- Glutathione Assay (GSH): Oxidative stress
Please also view the Mechanism of Action page.
Examples of Intestinal Toxicity Using EpiGLO™-Tox HT
The following examples were obtained using primary, human epithelial cells from the small intestines. All compounds were tested over the same dose range using EpiGLO™-Tox HT.
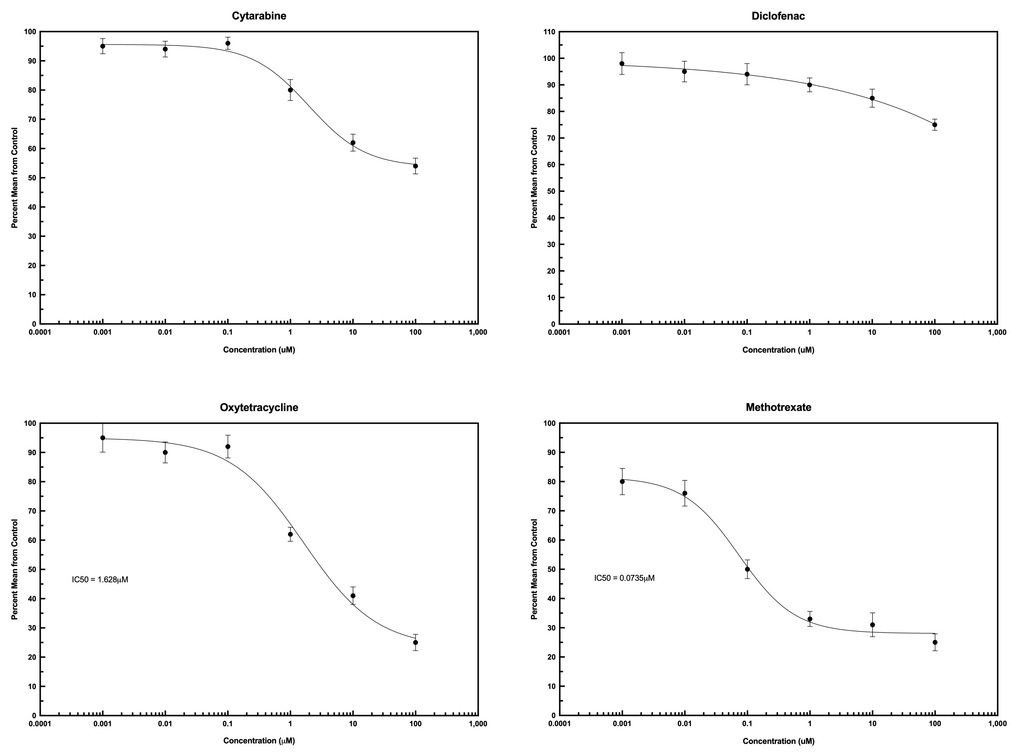

Comparing and Ranking Intestinal Toxicity with Other Biological Systems - ComparaTOX™
This unique in vitro toxicity testing platform called ComparaTOX™. It is based on the fact that other HemoGenix® toxicity assays all use a common readout, namely a standardized and validated ATP bioluminescence signal detection system. For example, HALO®-Tox HT, ImmunoGlo™-Tox HT, MSCGlo™-Tox HT, HepatoGlo™-Tox HT and NeuroGlo™-Tox HT. When combined together into the ComparaTOX™Platform, the results from each assay platform can be directly compared with each other. This allows the response to drugs and other agents to be ranked according to:
- Drug toxicity
- Cell type
- Species
Assay Kits for In-House Intestinal Toxicity Testing
You can perform in vitro intestinal toxicity testing in-house using assay kits sold by Preferred Cell Systems™. Please click on the links below to take you to the assay kit page on the Preferred Cell Systems website.
