Predictive Hematotoxicity /
Bone Marrow Toxicity
In Vitro High-Throughput Stem and Progenitor Cell Hematotoxicity Screening and/or Testing using HALO®-Tox HT
HemoGenix® is a Compliant Contract Research Service Supplier through Scientist.com
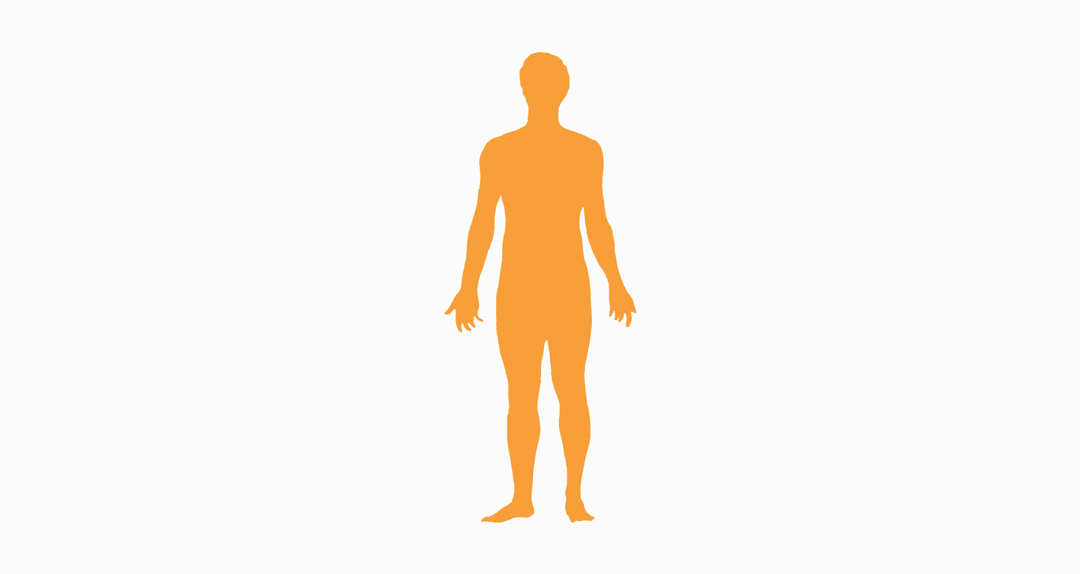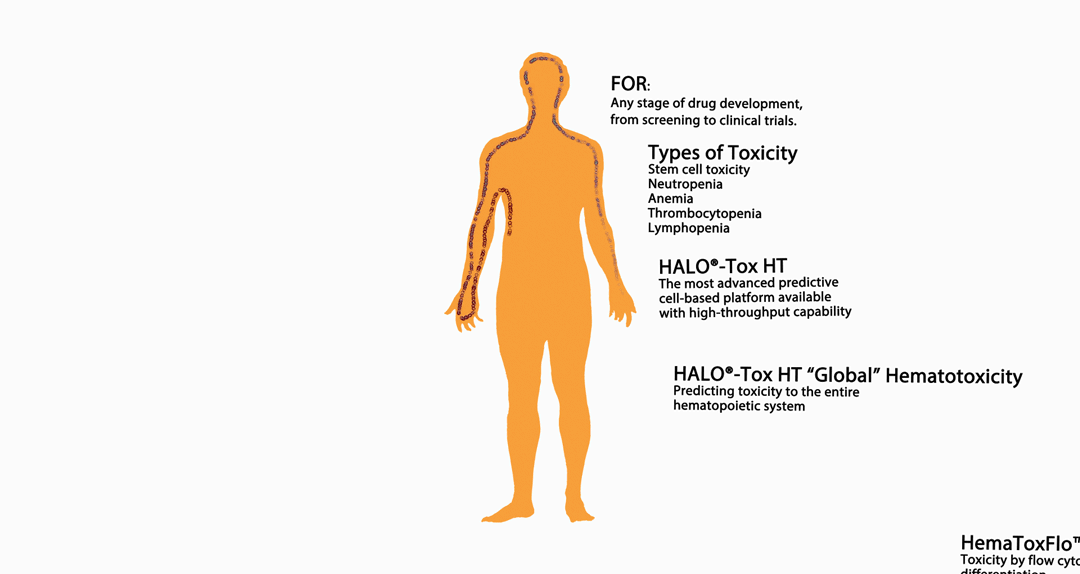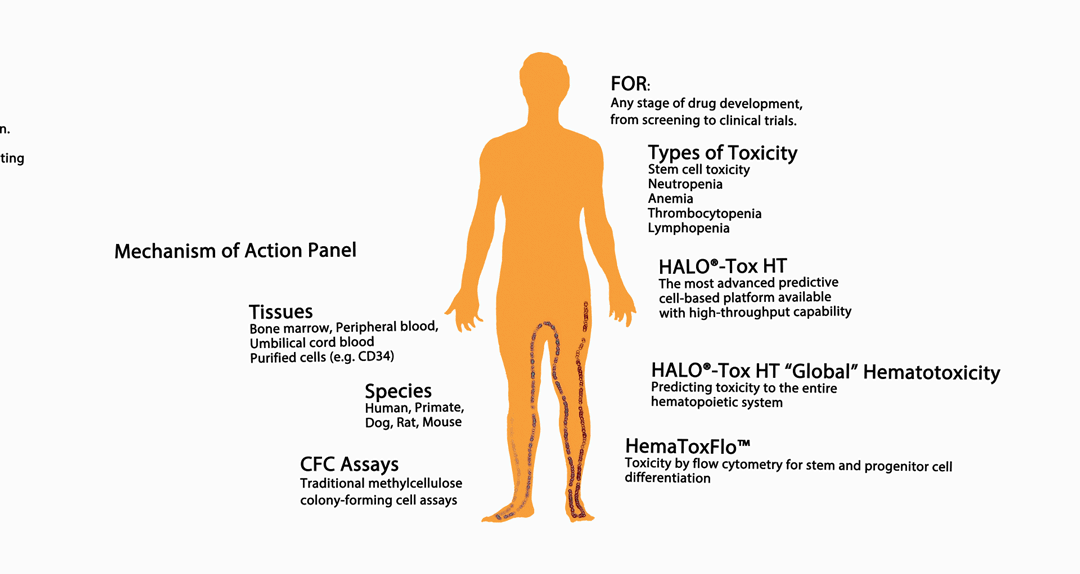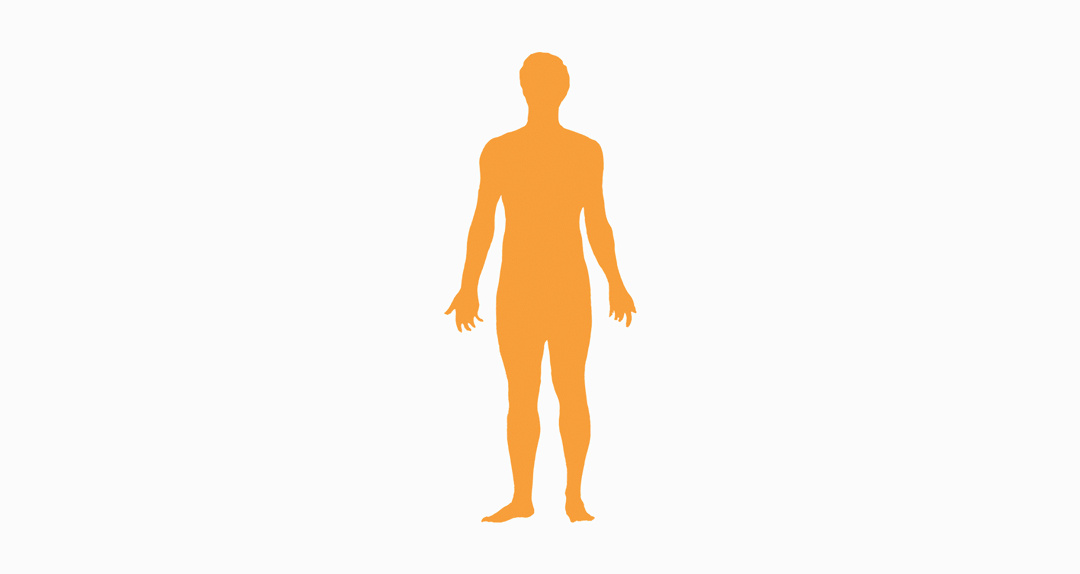
The infographic above provides an overview of HemoGenix® hematotoxicity / bone marrow toxicity capabilities.
For more information, please contact HemoGenix® directly at contractresearch@hemogenix.com or call (719) 264-6250. See our 100% ratings on the ThermoFisher Scientific Service Marketplace
Click on the arrows to expand the accordions below
An Introduction to Stem Cell Hematotoxicity / Bone Marrow Toxicity

The hematopoietic system is a vital and complex network responsible for the production of blood cells. This system includes bone marrow, blood cells, and the organs involved in hematopoiesis, such as the spleen and lymph nodes. The normal functioning of this system is crucial for maintaining a steady state of blood cell production in the body, ensuring adequate oxygen transport, immune defense, and hemostasis.
Types of Perturbations
Several factors can disrupt the hematopoietic system, leading to various health issues. These perturbations can be classified into several categories:
- Drugs: Certain medications, including chemotherapy agents, antibiotics, and immunosuppressants, can have profound effects on hematopoiesis. Chemotherapy drugs, for instance, target rapidly dividing cells, which include both cancer cells and healthy blood cells, leading to conditions such as anemia, leukopenia, and thrombocytopenia.
- Environmental Agents and Xenobiotics: Exposure to environmental toxins such as pesticides, heavy metals, and industrial chemicals can impair the hematopoietic system. For example, benzene exposure has been linked to bone marrow toxicity and an increased risk of leukemia.
- Food Additives: While most food additives are considered safe, some can potentially affect hematopoiesis. For example, excessive intake of certain preservatives or artificial coloring agents has been associated with allergic reactions and other immune responses that can indirectly influence blood cell production.
- Cosmetic Additives; Certain chemicals found in cosmetics, such as parabens and phthalates, are known to be endocrine disruptors. Although their direct impact on the hematopoietic system is less documented, their ability to interfere with hormonal balance can have downstream effects on blood cell production and function.
- Fuel Additives; Inhalation of substances from fuel additives, such as those found in gasoline and diesel, can lead to respiratory and systemic toxicity. Prolonged exposure to these substances has been associated with bone marrow suppression and an increased risk of hematological malignancies.
- Radiation Exposure; Ionizing radiation, including X-rays and gamma rays, can cause significant damage to the DNA of hematopoietic cells. This can lead to mutations, bone marrow failure, and an elevated risk of leukemia and other blood disorders.
Mechanisms of Hematopoietic / Immune Disruption
The impact of these perturbations on the hematopoietic system can occur through various mechanisms:
- Direct Cytotoxicity: Many perturbations cause direct damage to the bone marrow cells, leading to reduced production of one or more types of blood cells. This can result in conditions such as aplastic anemia, where the bone marrow fails to produce sufficient blood cells.
- Immune-Mediated Damage: Certain agents can trigger immune responses that target blood cells or bone marrow. For example, drug-induced hemolytic anemia occurs when the immune system mistakenly attacks red blood cells in response to a medication.
- Oxidative Stress: Exposure to toxins and radiation often results in the generation of reactive oxygen species (ROS), which can damage cellular components, including DNA, proteins, and lipids. This oxidative stress can impair the function and viability of hematopoietic stem cells.
- Endocrine Disruption: Substances that interfere with hormonal regulation can indirectly affect hematopoiesis. Hormones such as erythropoietin and thrombopoietin play critical roles in the production of red blood cells and platelets, respectively. Endocrine disruptors can alter the levels or activity of these hormones, leading to hematological imbalances.
Clinical Implications
The disruption of the hematopoietic system by various perturbations can lead to a range of clinical conditions:
- Anemia: A decrease in red blood cells or hemoglobin can lead to anemia, characterized by fatigue, weakness, and pallor. Anemia can result from direct bone marrow toxicity, nutrient deficiencies, or chronic diseases.
- Leukopenia: A reduction in white blood cells, particularly neutrophils, can increase susceptibility to infections. Leukopenia is commonly seen in patients undergoing chemotherapy or those exposed to radiation.
- Thrombocytopenia: A low platelet count can cause excessive bleeding and bruising. Thrombocytopenia can result from bone marrow suppression, autoimmune disorders, or certain medications.
- Hematological Malignancies: Chronic exposure to certain chemicals, radiation, and other perturbations can increase the risk of developing blood cancers such as leukemia, lymphoma, and myeloma.
The Hematopoietic System: The Role and Impact of Stem Cells
The hematopoietic system, like most biological systems in the body, is fundamentally a stem cell system. This system is unique in its potential to produce multiple cell types, each with distinct functions, from a single stem cell. This remarkable capability underscores the importance of the stem cell compartment, which is the cornerstone upon which all biological systems are built.
The Foundation of Biological Systems
For all biological systems, the stem cell compartment contains a spectrum of stem cell primitiveness or maturity. This diversity within the compartment is crucial for the development and maintenance of the body's various functions. The stem cell compartment's integrity is essential for the proper functioning of the biological system it supports. Any damage to this compartment, or subsequent compartments, can result in significant harm to the biological system and to the organism as a whole.
Understanding Stem Cell Hematotoxicity
Stem cell hematotoxicity refers to damage inflicted upon the stem cell compartment that subsequently affects the entire lympho-hematopoietic system. This system is responsible for the organization, regulation, and feedback mechanisms required to maintain a steady state condition. Hematotoxicity disrupts these delicate processes, leading to potential health risks and diseases.
Mechanisms of Hematotoxicity
Hematotoxicity can be caused by various factors, including exposure to certain chemicals, radiation, and pharmaceuticals. These agents can interfere with the normal functioning of stem cells, leading to their damage or destruction. The impact of hematotoxicity is profound because it not only affects the production of new blood cells but also disrupts the regulatory mechanisms that ensure a balanced and functional hematopoietic system.
Consequences of Hematotoxicity
The consequences of hematotoxicity are far-reaching. They can lead to conditions such as anemia, immunodeficiency, and increased susceptibility to infections and malignancies. The disruption of the stem cell compartment's integrity impairs the body's ability to regenerate and maintain healthy blood and immune cells, which are critical for overall health and well-being.
The Impact of Bone Marrow Toxicity
Bone marrow toxicity, often referred to as myelotoxicity, specifically denotes the toxicological damage to one or more parts of the lympho-hematopoietic system and the microenvironmental system that supports it. The bone marrow is the primary site of hematopoiesis, the process by which new blood cells are produced. Therefore, any damage to the bone marrow has a direct and immediate impact on the body's ability to generate blood cells.
Causes of Bone Marrow Toxicity
Bone marrow toxicity can result from exposure to chemotherapeutic agents, environmental toxins, radiation therapy, and certain viral infections. These factors can impair the bone marrow's ability to produce healthy blood cells, leading to a condition known as bone marrow suppression or aplasia. In severe cases, bone marrow toxicity can be life-threatening, necessitating urgent medical intervention.
- Clinical Manifestations: The clinical manifestations of bone marrow toxicity vary depending on the severity and extent of the damage. Common symptoms include fatigue, pallor, frequent infections, and easy bruising or bleeding. These symptoms arise from the reduced production of red blood cells, white blood cells, and platelets, respectively. Diagnosing and managing bone marrow toxicity requires a comprehensive understanding of the patient's medical history, potential exposure risks, and laboratory evaluations.
- Regenerative Medicine and Hematopoietic Stem Cells: Advances in regenerative medicine offer promising prospects for addressing stem cell hematotoxicity and bone marrow toxicity. Hematopoietic stem cell transplantation (HSCT) is a well-established therapeutic approach for treating various hematological disorders, including those caused by toxicity. Please click on this link to see more information.
The Interconnection of Hematotoxicity and Immunotoxicity: A Toxicological Perspective
From the viewpoint of toxicology, hematotoxicity and immunotoxicity are inseparable, since common stem cells give rise to both systems. If these stem cells are damaged, it inevitably affects both the hematopoietic and immune systems. This intrinsic connection underscores the importance of detecting the response of stem cells to potentially toxic agents, as it can provide predictive information for the rest of the lympho-hematopoietic system.
Hematotoxicity and Immunotoxicity: Definitions and Implications
- Hematotoxicity: Hematotoxicity refers to the toxic effect of substances on the blood and blood-forming tissues. It can lead to conditions such as anemia, leukopenia, thrombocytopenia, and aplastic anemia. The hematopoietic system relies on the bone marrow's ability to produce sufficient and functional blood cells, and any disruption can have severe consequences on overall health.
- Immunotoxicity: Immunotoxicity involves the adverse effects on the immune system, including suppression or overactivation of immune responses. It can result in increased susceptibility to infections, autoimmune diseases, and hypersensitivity reactions. The immune system plays a crucial role in defending the body against pathogens and maintaining homeostasis, so its impairment can lead to significant health issues.
The Role of Stem Cells in the Lympho-Hematopoietic System
Stem cells are undifferentiated cells capable of giving rise to various cell types. In the context of the lympho-hematopoietic system, hematopoietic stem cells (HSCs) found in the bone marrow differentiate into all blood cell lineages, including erythrocytes, leukocytes, and platelets, as well as cells of the immune system, such as T cells, B cells, and natural killer cells.
Common Origin of Hematopoietic and Immune Cells
Both hematopoietic and immune cells originate from HSCs. These multipotent stem cells undergo a complex process of differentiation and maturation to develop into specialized cells that perform distinct functions within the blood and immune systems. Given their shared origin, damage to HSCs can disrupt the production of both blood and immune cells.
Detecting Stem Cell Response to Toxic Agents
Predictive Information from Stem Cell Responses
Evaluating the response of HSCs to potentially toxic agents is critical for predicting the broader impact on the lympho-hematopoietic system. Substances that adversely affect HSCs can impair the production and function of progenitor cells, leading to downstream effects on both the hematopoietic and immune systems. By monitoring stem cell health and response, toxicologists can anticipate and mitigate potential hematotoxic and immunotoxic effects.
Techniques for Assessing Hematotoxic and Immunotoxic Effects
Several techniques are employed to assess the impact of toxic agents on stem cells and the lympho-hematopoietic system. These include:
- In vitro assays: Culturing HSCs in the presence of toxic substances to observe changes in cell viability, proliferation, and differentiation.
- Animal models: Using animal studies to evaluate the systemic effects of toxic agents on bone marrow and immune function.
- Biomarkers: Identifying specific biomarkers indicative of stem cell damage or dysfunction, such as changes in gene expression or protein levels.
Limitations of Conventional Pathology in Detecting Bone Marrow Toxicity and the Evolution of Advanced In Vitro Toxicity Testing Platforms
Conventional pathology has long been the cornerstone of detecting toxicity to bone marrow and other hematopoietic tissues during pre-clinical animal testing. However, these traditional methods often fall short in providing the predictive insight necessary to accurately determine drug-induced toxicity.
Advanced in vitro toxicity testing platforms have emerged as a more reliable alternative, offering greater precision and relevance.
Historical Context of In Vitro Toxicity Testing
During the late 1990s and early 2000s, significant advancements were made in the field of in vitro toxicity testing. The European Center for the Validation of Alternative Methods (ECVAM) played a pivotal role in this evolution, publishing numerous articles on the colony-forming cell (CFC) assay's use to predict drug-induced myelosuppression. Initial studies focused on the myelomonocytic (granulocyte-macrophage) lineage and later expanded to the megakaryocytic lineage. These studies, conducted across multiple locations, sought to validate the CFC assay for bone marrow toxicity.
The Colony-Forming Cell (CFC) Assay
The CFC assay has been a fundamental tool in studying bone marrow toxicity since its first publication in 1966. HemoGenix® was among the pioneering companies to offer this assay, and it continues to do so today. Despite its long-standing application, the methodology underlying the CFC assay has not undergone significant changes over the decades. While recombinant growth factors and cytokines and automated colony counting have been introduced, the assay's core methodology remains largely unchanged.
Challenges and Limitations of the CFC Assay
- Methylcellulose: One of the primary challenges associated with the CFC assay is its reliance on viscous methylcellulose to immobilize cells so that colonies of cells are formed. This approach is inherently error-prone, as methylcellulose cannot be dispensed accurately, leading to high coefficients of variation (CVs). Such variability hampers statistical relevance and the precise estimation of inhibitory concentration (IC) values.
- Proliferation vs. Differentiation: While the production of cell colonies necessitates the proliferation of cells, the CFC assay does not quantitatively measure cell proliferation. Instead, it assesses the differentiation capability of these cells. It is essential to recognize that proliferation and differentiation, albeit interconnected, are fundamentally distinct biological processes and, therefore, require two different readouts. The CFC assay focuses on the ability of cells within colonies to differentiate and mature, which allows researchers to identify the colonies' hematopoietic lineage. This differentiation helps distinguish between agents causing neutropenia, anemia, or thrombocytopenia.
- Microscopy is the Readout: Even with the introduction of expensive automated colony counting, the assay has remained the same with the same problematic errors associated with it. The type of colony still has to be enumerated, which is performed either manually or by algorithm-based counting of colonies using a microscope. This means that subjective and biased colony eneumeration is a major problem.
- Sensitivity Issues: Stem cells represent a minuscule fraction of the total bone marrow cellularity, typically less than 0.1%. Due to the low plating efficiency of the CFC assay, it struggles to reliably detect these rare populations. The assay's sensitivity is inadequate for identifying stem cell hematotoxicity, making it an unreliable and non-repeatable method for such purposes. Furthermore, many companies offering the CFC assay do not incorporate the complete range of growth factors necessary for detecting very primitive hematopoietic stem cells, such as CFC-GEMM. This omission further compounds the assay's limitations.
- Outdated Recommendations: Despite these significant drawbacks, the FDA and other regulatory agencies continue to recommend the CFC assay for detecting potential myelosuppressive effects of agents. This recommendation is based on decades-old data and does not account for advancements in stem cell research or modern technological developments. The reliance on outdated protocols highlights the need for updated guidelines that reflect current scientific understanding.
Modern Alternatives
In light of the CFC assay's limitations, researchers and laboratories are turning to more advanced methods for hematopoietic assessments. Technologies such as flow cytometry, single-cell RNA sequencing, and high-throughput screening offer greater sensitivity and accuracy in detecting rare cell populations. These techniques provide a more comprehensive view of cell behavior, including proliferation and differentiation, and are better suited for modern hematotoxicity studies.
Case Study: HemoGenix®
HemoGenix® is an example of a company that has recognized and addressed the limitations of the traditional CFC assay since 2002. By incorporating a broader spectrum of growth factors, HemoGenix® has developed assays that can detect very primitive hematopoietic stem cells more reliably. This approach demonstrates the potential for innovation in assay design and the importance of adopting updated methodologies. They leverage cutting-edge technologies and methodologies to overcome the inherent flaws of traditional assays like the CFC. By doing so, they offer a more accurate and statistically relevant assessment of bone marrow toxicity, paving the way for safer and more effective drug development.
HALO®-Tox HT: The Pinnacle of Hematotoxicity Screening and Testing: A Revolutionary Platform in Stem Cell and Bone Marrow Toxicity Testing
In 2002, HemoGenix® introduced a revolutionary platform with the aid of a grant from the National Cancer Institute. Known today as HALO®-Tox HT, this platform has redefined the landscape of stem cell hematotoxicity and bone marrow toxicity testing, establishing itself as the most advanced and reliable system available.
- The Genesis of HALO®-Tox HT: HemoGenix® embarked on a transformative journey with the launch of HALO®-Tox HT. This platform was designed to address critical needs in the biopharmaceutical industry, combining cutting-edge technology with unprecedented accuracy and sensitivity. The foundation of HALO®-Tox HT lies in its innovative Suspension Expansion Culture™ (SEC™) Technology. By making the conventional methods using methylcellulose and the CFC assay obsolete, SEC™ Technology represents a significant leap forward in cell culture techniques.
- Suspension Expansion Culture™ (SEC™) Technology: SEC™ Technology is at the heart of HALO®-Tox HT's success. Traditional methods like the CFC assay, which relied heavily on methylcellulose, have been surpassed by the advanced capabilities of SEC™. This technology allows for the expansion and culture of cells in suspension, providing a more accurate representation of hematopoietic environments. The ability to culture cells in suspension enhances the platform's precision and efficiency, ensuring that the results obtained are both reliable and repeatable. The move away from methylcellulose is not just a technological advancement; it represents a paradigm shift in how stem cell and bone marrow toxicity testing is conducted. The SEC™ Technology incorporated into HALO®-Tox HT allows for a more dynamic and versatile approach, accommodating the complexities of various cell types and their interactions.
- High-Throughput Capabilities and Liquid Handler Instrumentation: One of the standout features of HALO®-Tox HT is its high-throughput capability. The platform is designed to integrate seamlessly with liquid handler instrumentation, enabling the processing of large sample volumes with unparalleled accuracy. This high-throughput capability is crucial for early drug development and pre-clinical animal studies, where the ability to process and analyze numerous samples efficiently can significantly accelerate the research timeline. The use of liquid handler instrumentation not only enhances throughput but also ensures precision. The automation provided by these instruments minimizes human error, resulting in more consistent and reliable data. This is particularly important in toxicity testing, where even minor discrepancies can lead to significant variations in results.
- ATP Bioluminescence Technology: The incorporation of ATP bioluminescence technology into HALO®-Tox HT has further cemented its position as the leading platform in hematotoxicity testing. ATP bioluminescence is the most advanced and sensitive non-radioactive signal detection system available today. By measuring the levels of ATP (adenosine triphosphate) within cells, this technology provides a direct indication of cell viability and metabolic activity. The sensitivity of ATP bioluminescence allows for the detection and measurement of rare stem cell populations that were previously difficult to analyze. This capability is critical for understanding the potential toxicity of new drug compounds on specific cell types, providing valuable insights that can guide the development of safer and more effective therapies.
- Standardization and FDA Bioanalytical Method Validation: To ensure the reliability and repeatability of results, HALO®-Tox HT incorporates rigorous standardization and FDA bioanalytical method validation. This validation process is essential for meeting the stringent requirements of the biopharmaceutical industry. By adhering to these standards, HALO®-Tox HT provides a level of assurance that the data obtained from its tests can be trusted for regulatory submissions and decision-making processes. The standardization of HALO®-Tox HT also facilitates comparison across different studies and laboratories, enhancing the reproducibility of findings. This is particularly important in the context of drug development, where consistent results are necessary to advance compounds through the various stages of testing and approval.
- High (>80%) In Vitro to In Vivo Concordance: One of the key metrics for evaluating the effectiveness of a toxicity testing platform is its concordance between in vitro (laboratory) and in vivo (live organism) results. HALO®-Tox HT boasts a high concordance rate of over 80%, making it the most trusted platform for hematotoxicity screening and testing. This high level of concordance ensures that the results obtained from in vitro tests are predictive of the outcomes observed in vivo, providing researchers with confidence in their data. The ability to accurately predict in vivo responses from in vitro tests is crucial for early drug development. It allows researchers to identify potential toxicities before advancing compounds to animal studies, reducing the risk of adverse effects and improving the overall efficiency of the drug development process.
- Applications Across Drug Development and Pre-Clinical Studies: HALO®-Tox HT's versatility extends across various stages of drug development, from early screening to pre-clinical studies. In the early stages, the platform is used to screen potential drug candidates for hematotoxicity, identifying compounds that may have adverse effects on stem cell populations. This early screening helps to prioritize compounds with the best safety profiles, reducing the likelihood of late-stage failures.
- During pre-clinical studies, HALO®-Tox HT provides detailed insights into the potential toxicities of drug candidates. By evaluating the effects of compounds on bone marrow and hematopoietic stem cells, researchers can identify dose-limiting toxicities and optimize dosing regimens. This information is critical for designing safe and effective clinical trials.
Conclusion
Since its launch in 2002, HALO®-Tox HT has revolutionized the field of hematotoxicity and bone marrow toxicity testing. With its advanced SEC™ Technology, high-throughput capabilities, and incorporation of ATP bioluminescence, the platform offers unparalleled accuracy, sensitivity, and precision. The rigorous standardization and high concordance rate further enhance its reliability, making it the most trusted platform for toxicity screening and testing in the biopharmaceutical industry.
As drug development continues to evolve, HALO®-Tox HT remains at the forefront, providing researchers with the tools they need to develop safer and more effective therapies. The platform's ability to detect and measure rare stem cell populations, combined with its high-throughput capabilities, ensures that it will continue to play a vital role in advancing our understanding of drug toxicity and improving patient outcomes.
Types of Toxicity Studies Available
The majority of studies performed by HemoGenix® incorporate a Complete Service, Full Report that can be used for an IND application. More recently, Sponsors have requested a more streamlined study and/or studies involving high-throughput screening. For this reason, HemoGenix® provides 3 types of study format.
- Complete Service, Full Report Study: Fully customized study that includes the Study Plan, Draft Text with discussion, interpretation and conclusion of results and, Final Text Report with QA audit. Our Full Report Study Template can be included in a regulatory agency filing application or we can use the Sponsor's Template.
- Rapid Toxicity Study Report: A customized study that includes the Study Plan and full protocol, raw results and graphical data in a single Excel Workbook. No formal text report and no QA audit is performed. This type of study is designed for early drug toxicity screening and development. No discussions, interpretation or conclusions are provided.
Species and Tissues Available for Toxicity Screening and/or Testing Assays
All instrument-based assay platforms and methylcellulose CFC assays are available for use with the following species:
- Human
- Non-human primate (Macaca fascicularis or cynomologus (Cyno) and Macaca mulatta (Rhesus)
- Dog
- Rat (various strains)
- Mouse (various strains)
Other species available and which might be of interest include:
- Minipig
- Pig
- Sheep
- Horse
Tissues and Purified Cell Populations Available for Testing
- Bone marrow (all species)
- Peripheral blood (human and large animal species; please inquire about rodents)
- Umbilical cord blood (human)
- Purified stem cell populations (e.g. human CD34 , CD133 cells)
- Purified human B lymphopoietic cells
- Other hematopoietic organs from animals (e.g. spleen, fetal liver, yolk sac)
PLEASE NOTE: We often are asked whether we can perform assays, especially methylcellulose CFC assays, using "whole" or red blood cell or plasma-reduced bone marrow, cord blood or peripheral blood, since this is provided by other companies. We DO NOT suggest or recommend using this type of tissue preparation for any toxicity assays, let alone quality or potency assays. Using these preparations may result in a less costly study, but the assays will lack sensitivity, accuracy, precision, reliability and reproducibility causing a serious underestimation of the results leading to potential misinterpretation and conclusion of the data. It is for this reason why we highly recommend a minimum mononuclear cell (MNC) fractionation for most species so as not to loose important cellular information.
HALO®-Tox HT: Hematotoxicity Screening / Testing for Compounds Affecting Cell Proliferation
Please Note that all 3 study types, (1) Complete Service, Full Report, (2) Rapid Toxicity Study and, (3) Screening Study are available using HALO®-Tox HT.
HALO®-Tox HT is a proprietary, fully standardized, high-throughput toxicity assay platform that has been validated according to FDA Bioanalytical Method Validation Guidelines. It incorporates two technologies:
- Suspension Expansion Culture™ (SEC™) Technology that allows (a) standardization and validation, (b) high sensitivity and accuracy, (c) high reliability and reproducibility, (d) rapid assay completion (5-7 days) and, (e) high-throughput capability. Indeed, HemoGenix® is the only company worldwide that provides high-throughput hematotoxicity testing. In contrast to the traditional colony-forming assay (CFC), HALO®-Tox HT does not employ methylcellulose and therefore obviates many of the drawbacks of the CFC assay (see below).
- ATP Bioluminescence Technology. Measuring intracellular ATP using a luciferin/luciferase bioluminescence output has been employed by the biopharmaceutical industry for many years. HemoGenix® has taken this technology to a higher level by providing standardization, validation and measurement assurance parameters for all contract research studies.
The nomeclature used to describe different cell populations of the lympho-hematopoietic systems is often confusing. In addition, although HALO® incorporates similar growth factor cocktails to detect different populations, colonies of cells are not produced, because HALO®-Tox HT incorporates advanced Suspension Expansion Culture™ (SEC™) Technology that obviates the use of methylcellulose and allows for high-throughput screening. Since the cell populations are detected in suspension culture, a different nomenclature is used to describe the populations: "SC" means "stem cell" and "P" means "progenitor cell" for all populations detected using HALO®-Tox HT.
| Stem Cell Type |
HALO® Stem Cell($) Designation |
Growth Factor Cocktail |
Pathways |
Predictive Toxicity |
HALO®-Tox HT Screening or Testing |
|---|---|---|---|---|---|
|
Quiescent, primitive lympho-hematopoietic stem cell: High Proliferative Potential - Stem and Progenitor Cell |
SC-HPP 1 |
IL-3, IL-6, SCF, TPO, Flt3-L | Lympho-Hematopoietic | Yes | Testing only |
| Induced, primitive lympho-hematopoietic stem cell: High Proliferative Potential - Stem and Progenitor Cell |
SC-HPP 2 |
EPO, GM-CFC, IL-2, IL-3, IL-6, IL-7, SCF, TPO, Flt3-L | Lympho-Hematopoietic |
Anemia Neutropenia Thrombo-cytopenia Lymphopenia |
384-well HT screening(*) and normal testing |
| Primitive hematopoietic stem cell: Granulocyte, Erythroid, Macrophage, Megakaryocyte |
SC-GEMM 1 |
EPO, GM-CFC, IL-3, IL-6, SCF, TPO, Flt3-L | Hematopoietic only |
Anemia Neutropenia Thrombo-cytopenia |
384-well HT screening(*) and normal testing |
(*) Part of the HemoGenix® ComparaTOX™ HT Platform for the rapid screening of compounds in multiples of 5. Please contact HemoGenix® for more nformation.
($) Other stem cell populations are also available. Please contact HemoGenix™ for more information.
|
Progenitor Cell Type |
HALO® Progenitor Cell Designation |
Growth Factor Cocktail | Pathways Detected | Predictive Toxicity |
HALO®-Tox HT Screening or Testing |
|---|---|---|---|---|---|
|
Primitive erythropoietic progenitor cell: Stem cells induced into the erythropoietic lineage |
P-BFU 1 |
EPO, IL-3, SCF | Erythropoietic lineage | Anemia | 384-well HT screening(*) and normal testing |
|
Primitive erythropoietic progenitor cell: Primitive erythropoietic progenitors already in compartment |
P-BFU 2 |
EPO alone | Erythropoietic lineage | Anemia | Testing only |
|
Primitive granulocyte- macrophage (GM) progenitor: Stem cells induced into the GM lineage |
P-GM 1 |
GM-CSF, IL-3, SCF |
Granulocyte /macrophage lineage |
Neutropenia | 384-well HT screening(*) and normal testing |
|
Primitive granulocyte- macrophage progenitor: Primitive GM progenitors already in compartment |
P-GM 2 |
GM-CSF, G-CSF, IL-3, SCF |
Granulocyte /macrophage lineage |
Neutropenia | Testing only |
|
Primitive megakaryocyte progenitor: Stem cells induced into the megakaryopoietic lineage |
P-Mk 1 |
TPO, IL-3, SCF |
Megakaryo-poietic lineage | Thrombo-cytopenia | 384-well HT screening(*) and normal testing |
|
Primitive T-lymphocyte progenitor: Stem cells induced into the T-cell lineage |
P-Tcell 3 |
IL-2 or cocktail |
T-cell lineage | Lymphopenia | 384-well HT screening(*) and normal testing |
|
Primitive B-lymphocyte progenitor: Stem cells induced into the B-cell lineage |
P-Bcell 2 |
IL-4 + cocktail |
B-cell lineage | Lymphopenia | 384-well HT screening(*) and normal testing |
(*) Part of the HemoGenix® ComparaTOX™ HT Platform for the rapid screening of compounds in multiples of 5. Please contact HemoGenix® for more nformation.
HALO®-Tox HT "Global" Lympho-Hematotoxicity Testing
In addition to screening or testing individual cell populations using HALO®-Tox HT, HemoGenix® has developed a "Global" Toxicity Assay Platform for testing compounds on multiple cell populations simultaneously to provide a more detailed "global" assessment of the lympho-hematopoietic lineages affected by potential toxicity.
The HALO®-Tox HT "Global" Platform is available either as (1) A Complete Service, Full Report, or (2) A Rapid Toxicity Study.
HALO®-Tox HT "Global" Contract Services are available for the following species:
- Human
- Primate (Cyno and Rhesus)
- Dog
- Rat
- Mouse
There are three (3) options for the HALO®-Tox HT "Global" Platform. These are:
1. HALO®-Tox HT "Global" 4-Population Assay that mesures the response to:
- SC-GEMM 1
- P-BFU 1
- P-GM 1
- P-Mk 1
2. HALO®-Tox HT "Global" 5-Population Assay that measures the response to:
- SC-HPP 1
- SC-GEMM 1
- P-BFU 1
- P-GM 1
- P-Mk 1
3. HALO®-Tox HT "Global" 7-Population Assay that measures the response to:
- SC-HPP 1
- SC-GEMM 1
- P-BFU 1
- P-GM 1
- P-Mk 1
- P-Tcell 3
- P-Bcell 2*
(*) This population requires that a purified population of P-Bcell are used.
Validation of HALO®-Tox HT as a Cytotoxicity Assay
Validation of HALO®-Tox HT as a Cytotoxicity Assay
Besides multiple biopharmaceutical companies validating HALO® either in-house or by contract services, HALO® has been validated against the Registry of Cytotoxicity Prediction Model. The Registry of Cytotoxicity contains a list of compounds for which the LD50 values in rats or mice have been documented. If the LD50 for a group of reference compounds is plotted against the IC50 values obtained from an in vitro assay, in this case HALO®-Tox HT, the individual points will lie on a linear regression with specific parameters. This validates the assay as a cytotoxicity assay. Thus, HALO®-Tox HT has been validated against the Registry of Cytotoxicity.
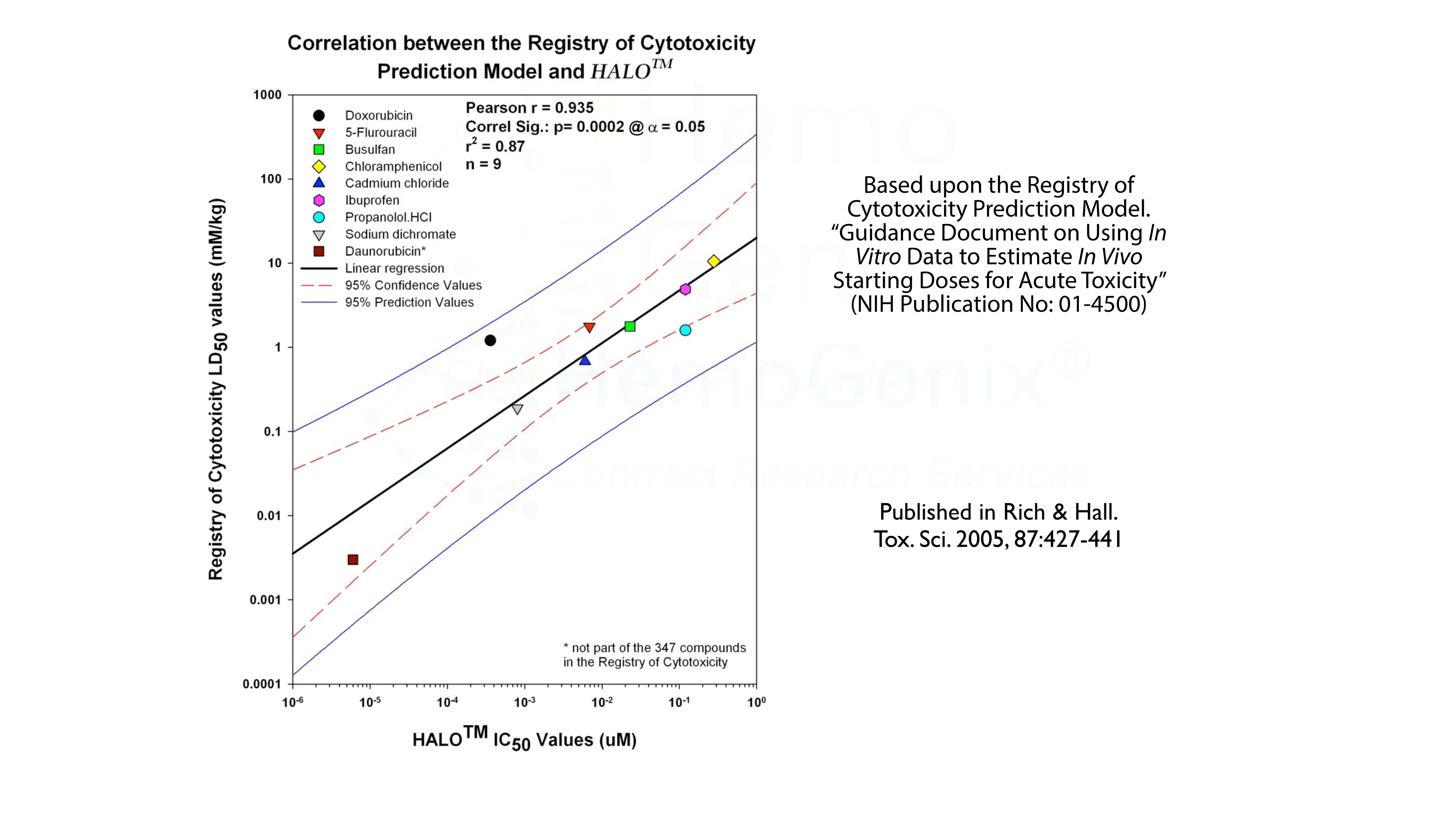
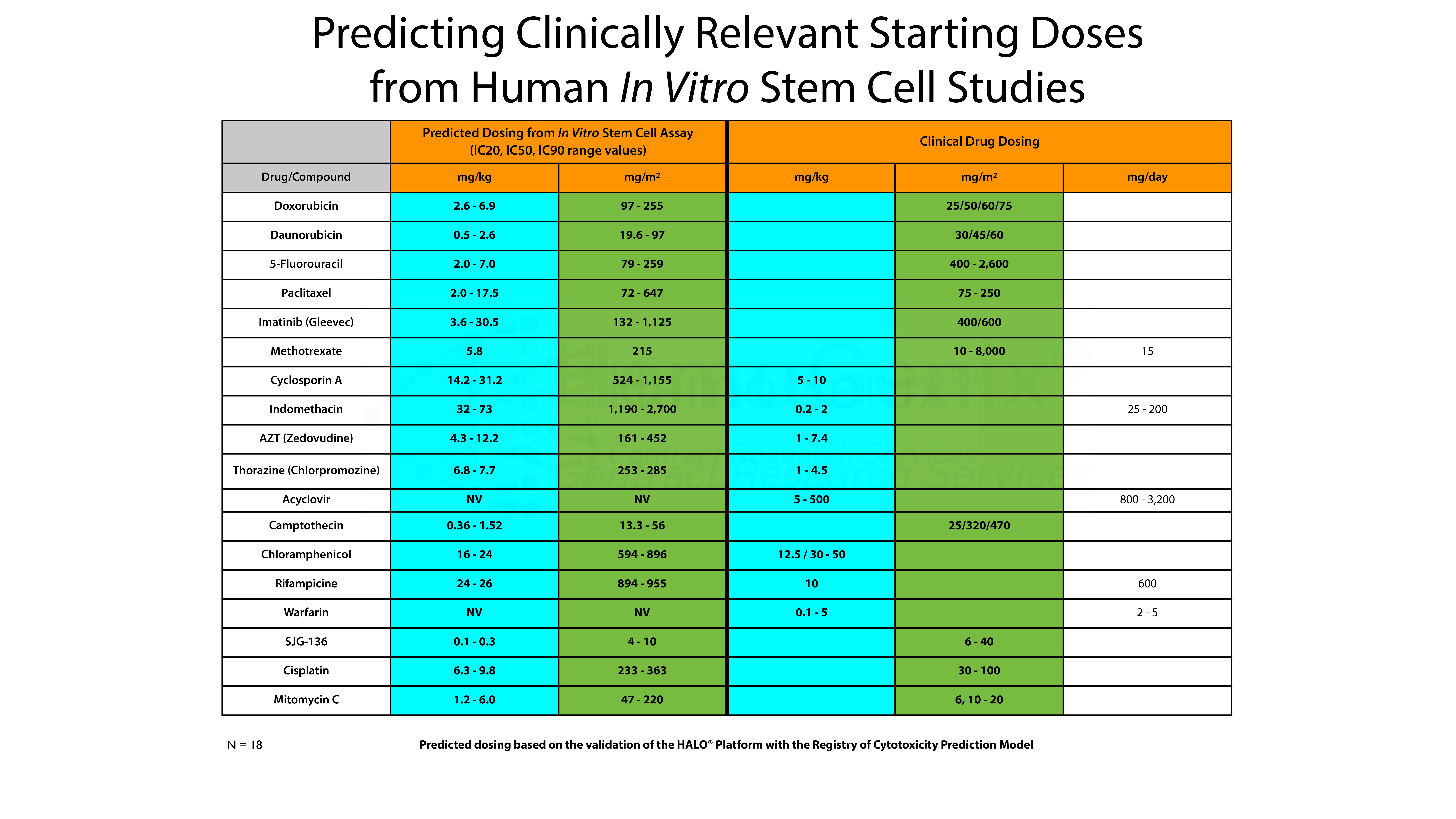
However, there is an even more interesting aspect. This is shown in the table below. Using the Registry of Cytotoxicity Prediction Model, it is possible to convert in vitro IC50 values into clinically relevant doses, either in mg/kg, mg/m2 or as mg/day. The conversion of in vitro IC values obtained using HALO®-Tox HT have been converted into clinically relevent doses based on the Registry of Cytotoxicity Prediction Model. In the majority of cases, the predicted in vitro values correspond to the dose ranges used in the clinic to treat various forms of human cancer. That is, the IC50 values derived from HALO®-Tox HT are, in the majority of cases, in the same order of magnitude as those used for treatment. It should be noted that this model can also be used to to predict the starting doses for pre-clinical animal studies.
Validation and Measurement Assurance Parameters for HALO®-Tox HT
HALO®-Tox HT has been validated according to FDA Bioanalytical Method Validation Guidelines which are summarized below.
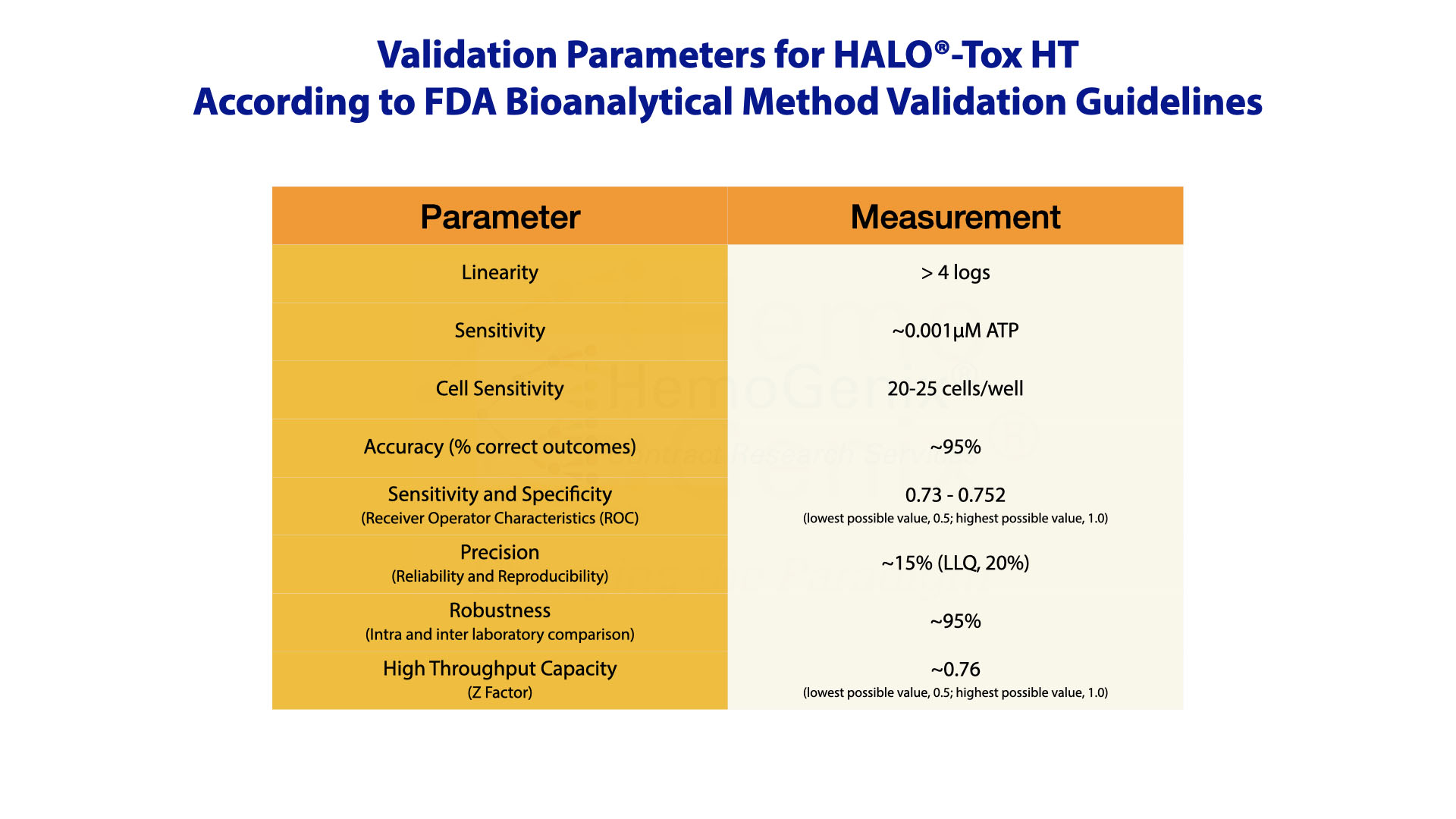
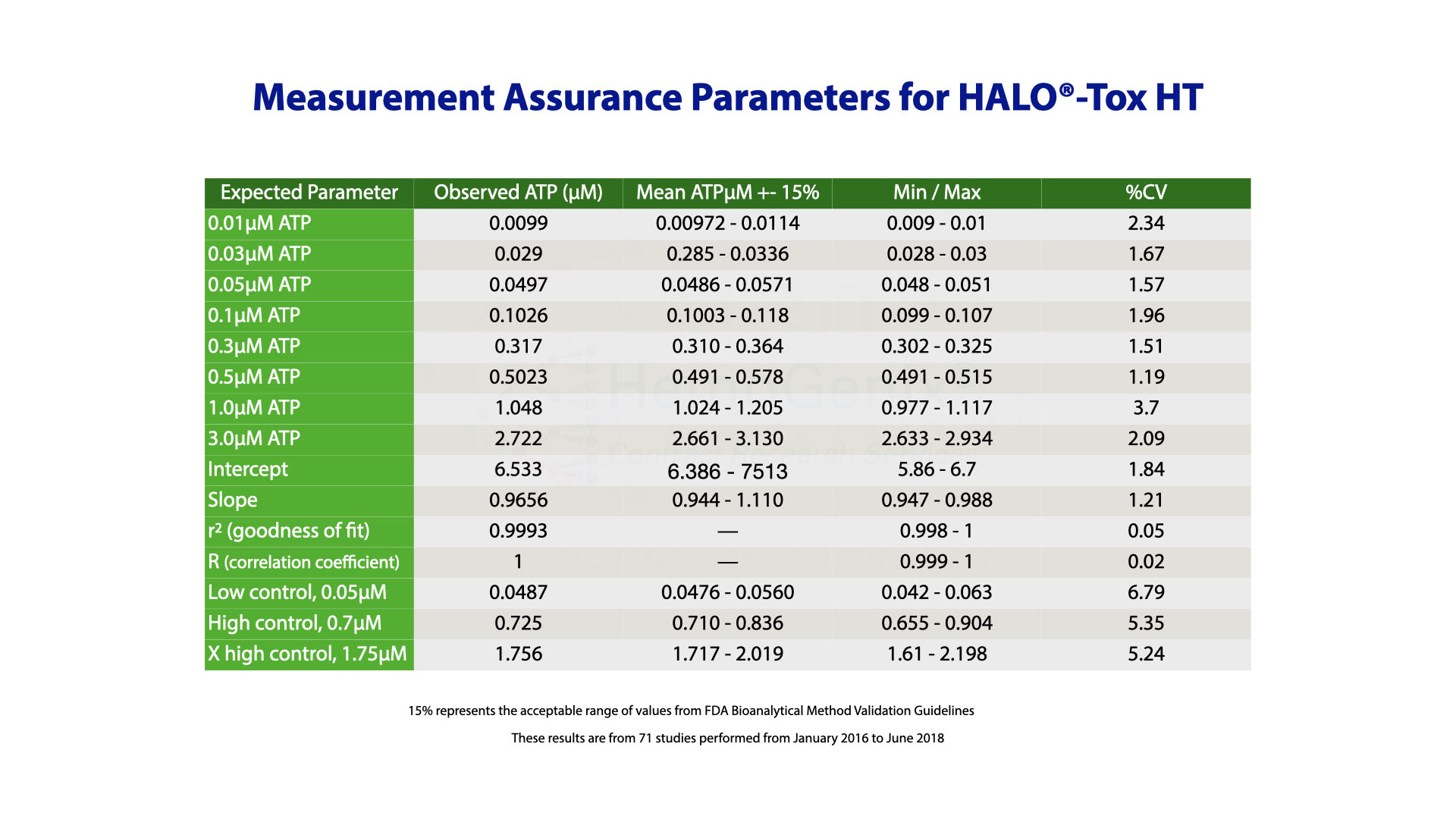
Validation of the HALO®-Tox HT Platform was only possible by incorporating controls to calibrate the assay and standards to ensure that the assay works correctly prior to processing and determining samples.
After assay validation, it was then possible to establish measurement assurance parameters from historical data that ensure results are trustworthy.
Example Applications Using HALO®-Tox HT
Example 1. HALO®-Tox HT and Multiple Donors - A Case for Reproducibility
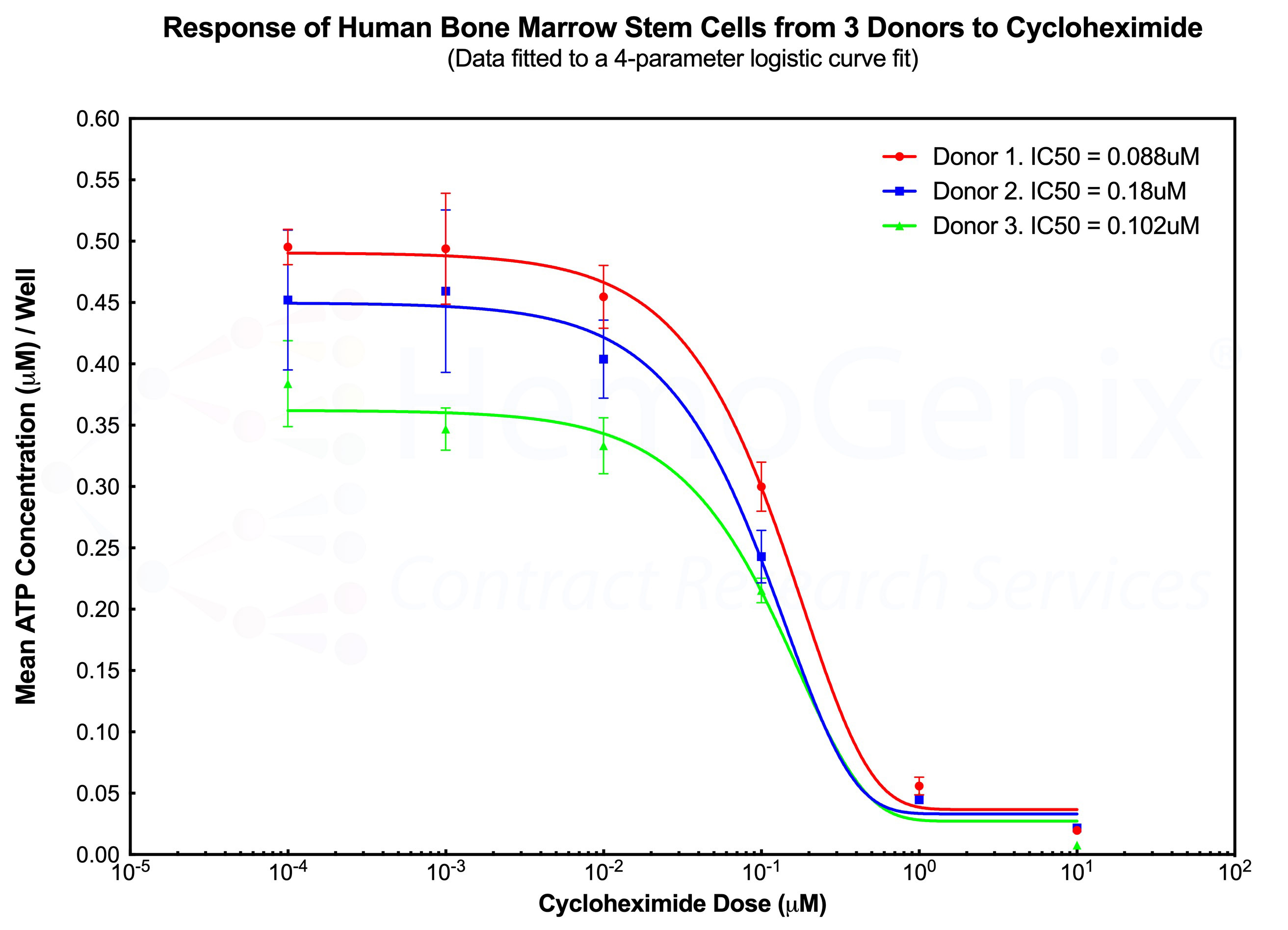
The diagram below shows the response of primitive human hematopoietic stem cells (SC-GEMM 1) to cycloheximide by 3 separate human donors performed on different days. Although each donor exhbits a different number of responding cells, as indicated by the different starting ATP concentrations at the lowest cycloheximide dose, the shape of the dose response curve and the IC50 values are very similar between each donor. This example demonstrates the high degree of reproducibility using HALO®-Tox HT. The example also illustrates that, in the majority of cases, a single donor is sufficient to obtain reliable results, thereby significantly reducing the cost of a study.
Example 2. Species Comparison
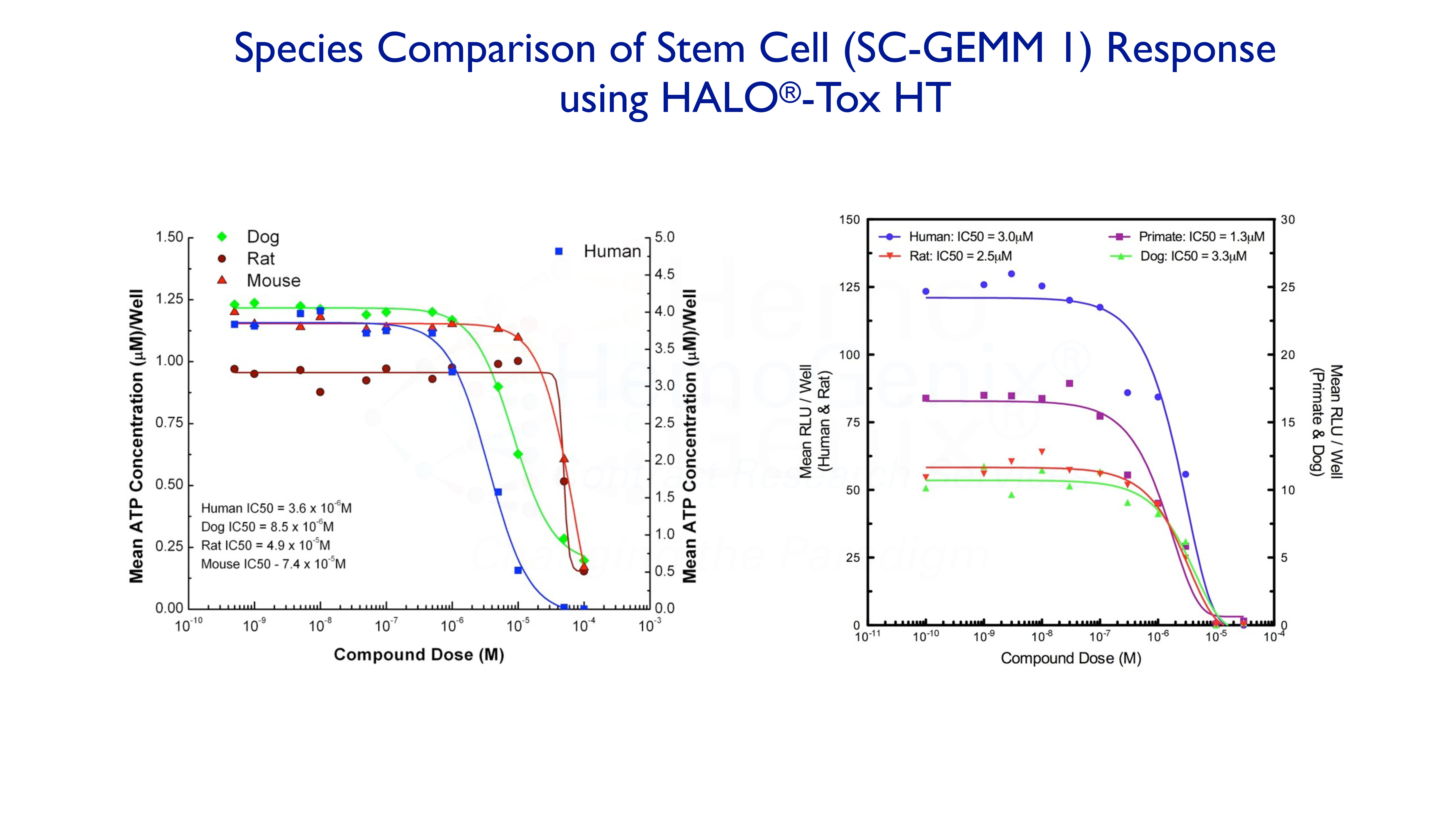
It is often the case that one compound will exhibit a different response in different species. The graphs below demonstrate how this may, or may not, occur. Although HALO®-Tox HT can predict many aspects of potential hematotoxicity, it cannot, as yet, predict differences in response between species.
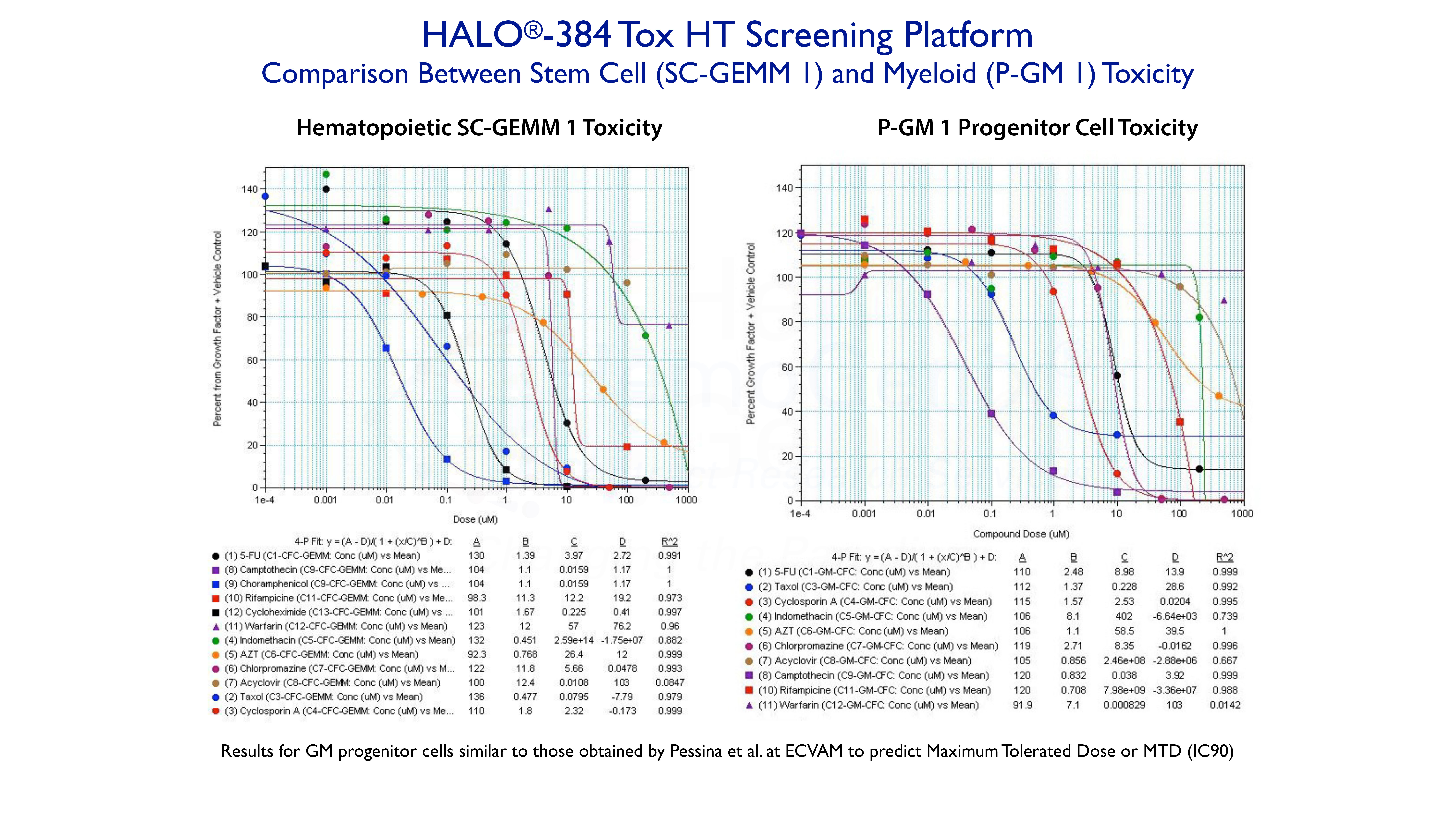
Example 3. High-Throughput Screening Using HALO®-384 Tox HT
The following shows a study in which up to 12 compounds, with different targets, were tested on human hematopoietic stem cells (SC-GEMM1 ) and granulocyte-macrophage progenitor cells (P-GM 1) using HALO®-384 Tox HT high-throughput screening platform. All of the dose response curves are fitted to a 4-parameter logistic curve fit using SoftMax Pro software. The approximate IC50 values are prvided by Parameter C.
The compounds shown for the P-GM 1 response were the same as those used by Pessina and coworkers at ECVAM to obtain the maximum tolerated dose (MTD) at IC90, in order to try and validate the colony-forming unit (CFU, see below) assay for toxicity testing. Although results were similar between the assays, HALO®-384 Tox HT is not only more rapid, accurate and reliable, but can also be used to rapidly rank compounds based on compound type, cell type and species. HALO®-Tox HT is part of the HemoGenix® ComparaTOX™ Platform.
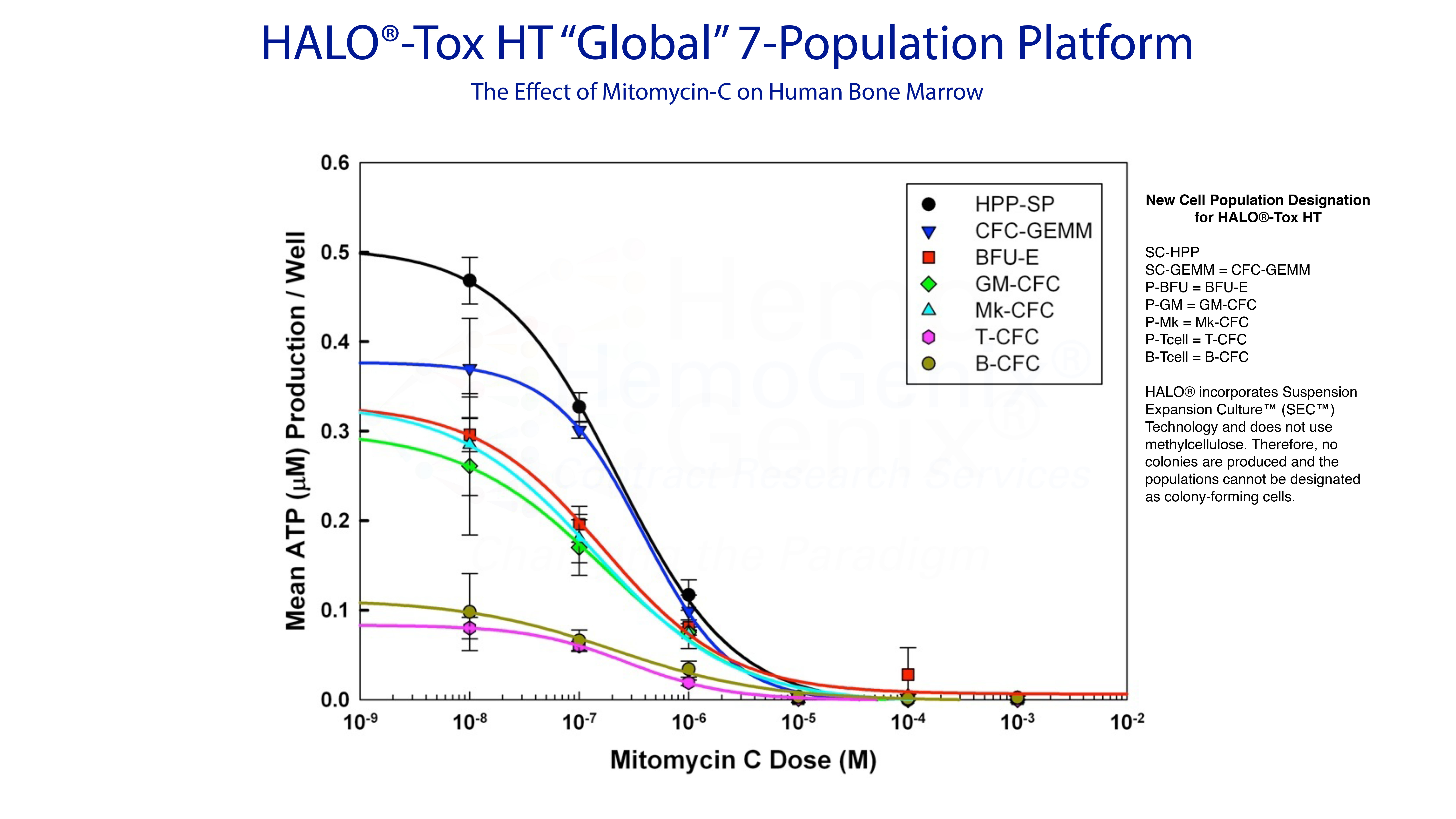
Example 4. Predicting Hematotoxicity Using HALO®-Tox HT "Global"
The HALO®-Tox HT "Global" Platform provides all the information to predict hematotoxicity for different compound types and species. As described above, there are 3 HALO®-Tox HT "Global" Platforms:
- HALO®-Tox HT "Global" 4-Population
- HALO®-Tox HT "Global" 5-Population
- HALO®-Tox HT "Global" 7-Population
The example below shows an example of the HALO®-Tox HT "Gloabl" 7-Population Platform for Mitomycin-C using huamn bone marrow mononuclear cells (MNC) as the target cell population. Notice that there are 3 groups of response, namely (1) the stem cell response, (2) the hematopoietic progenitor cell response and, (3) the lymphopoietic response.
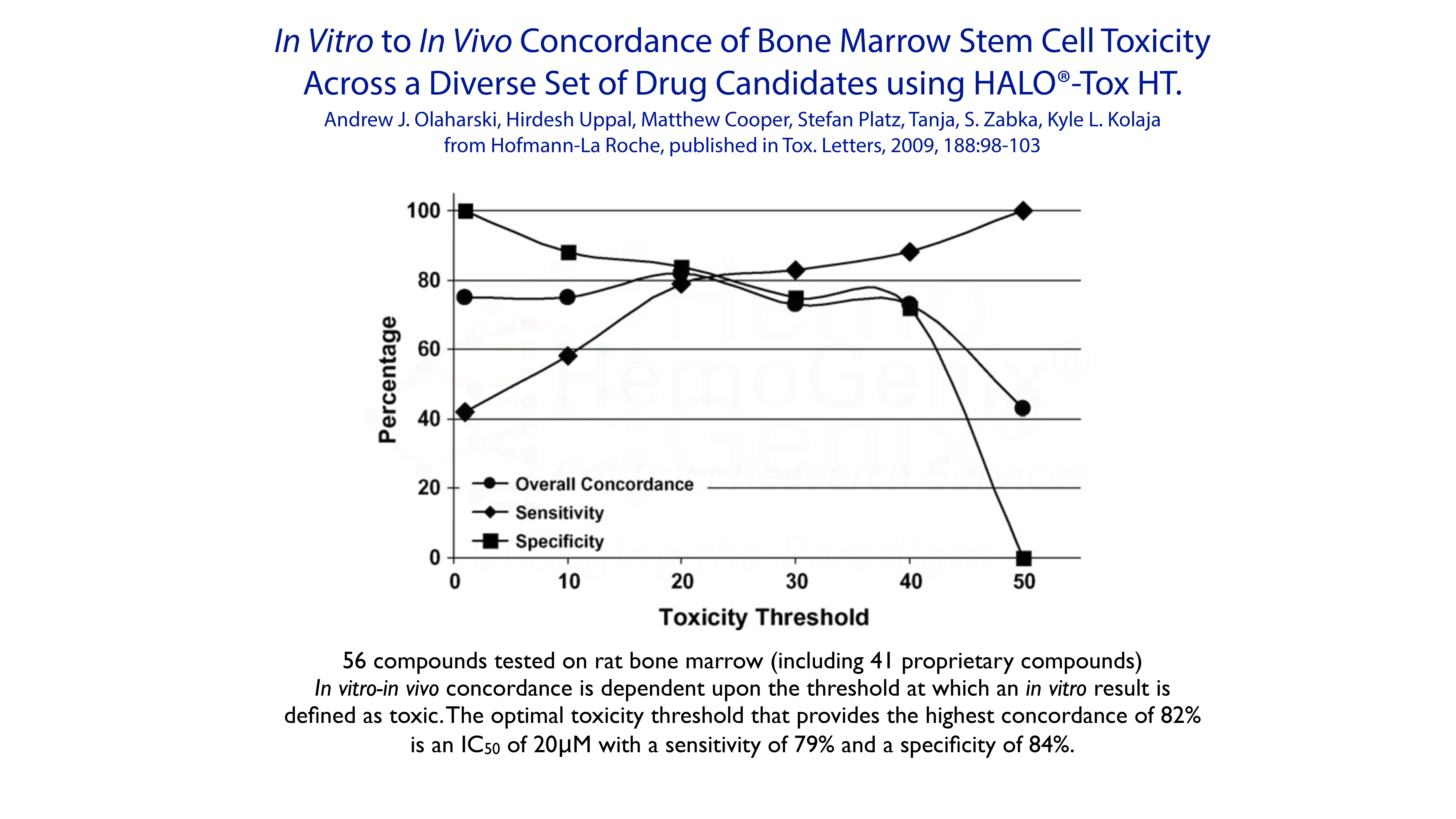
Example 5. In Vitro to In Vivo Concordance
The ability to predict the in vivo response from in vitro data has been one of the foundations for using in vitro assays to measure and determine potential toxicity of drugs and xenobiotics. In 2009, Olaharski and his colleagues published data showing that HALO®-Tox HT provided an 82% concordance with in vivo data from the rat. However, it should be noted that based on decades of publications, an extremely high concordance between in vitro and in vivo data for for multiple hematopoietic applications has been shown.
HemaToxFlow™ - Flow Cytometric Hematotoxicity Testing
HemaToxFlow™ is an in vitro flow cytometric assay used to determine potential hematotoxicity of stem and progenitor cells from human and mouse tissues. Since HemaToxFlow™ uses CD markers to define specific differentiated cell populations, it can replace the use of colony-forming cell (CFC) assays which relies on the ability of primitive cells to differentiate and mature.
For human studies, HemaToxFlow™ utilizes mononuclear cells (MNC), CD34+ or CD133+ cells from:
- Bone marrow
- Umbilical cord blood
- Peripheral blood (normal or mobilized).
For murine studies, fractionated and purified cells can be used, but are not a requirement. Murine target cells:
- Bone marrow
- Spleen
- Fetal liver.
HemaToxFlow™ reagents are used to stimulate the following defined cell populations from both species:
- SC-GEMM 1: Primitive hematopoietic stem cell
- P-BFU 1: Primitive erythropoietic progenitor cell
- P-GM 1: Primitive granulocyte-macrophage progenitor cell
- P-Mk 1: Primitive megakaryopoietic progenitor cell.
Method Summary
HemaToxFlow™ is performed in 96-well plates.
- Cell suspensions are prepared and adjusted to the correct final concentration.
- Test compounds are dissolved and serial dilutions prepared.
- HemaToxFlow™ Reagent for the specific cell population or populations to be analyzed are dispensed into the 96-well plate(s) using a liquid handler.
- This is followed by the addition of the cell suspension to each well.
- The test compound or compounds represent the final addition, also performed by a liquid handler.
- The plates are incubated in a 37 oC incubator for 4-5 days for murine cells and 5-7 days for human cells.
- After incubation, the cells in the plates are centrifuged and the supernatant removed and discarded.
- Pre-diluted, specific fluorophore-conjugated antibodies are then added in panels to each well so that different cell populations can be detected. Counting bead are also added to determine cell number.
- The cells in each well of the plate are then analyzed by flow cytometry using a Beckman Coulter Cytoflex flow cytometer.
- Cell counts are ploted against the drug dose and a 4-parameter logistic curve fit is used to determine IC values.
- If required, HemaToxFlow™ can be verified against HALO™-Tox HT
The table below shows the CD antibodies used to detect different cell populations from different human and mouse using the HemaToxFlow™ assay.
| HemaToxFlow™ Assay | Antibodies | Cell Population Detected |
|---|---|---|
| Human Stem Cells* | CD34 CD133 |
Hematopoietic stem cells (Additional antibodies can be added to determine lineage-specific differentiated cells). |
| Human Erythroid Cells | CD235a (GlyA) | Erythroid cells |
| Human Myelod Cells | CD13 CD14 CD15 |
Myelod progenitor cells Monocytes Granulocytes |
| Human Megakaryocytes | CD41 CD61 |
Megakaryocytes Platelets |
| Murine Stem Cells* | CD34 | Hematopoietic stem cells |
| Murine Erythroid Cells | CD235a Ter-119 |
Erythroid cells |
| Murine Myeloid Cells | Mac-1/CD11b F4/80 CD66b Gr-1/Ly6G Ly6C |
Monocytes/macrophages Monocytes/macrophages Granulocytes Granulocytes Granulocytes |
| Murine Megakaryocytic | CD41 CD61 |
Megakaryocytes/platelets Megakaryocytes/platelets |
(*) Due to the fact that the SC-GEMM 1 population is stimulated with a growth factor cocktail that produces lineage-specific hematopoietic cells, the CD antigen markers used for progenitor cell differentiation can also be used to determine differentiated cells produced from the stem cell population.
Methylcellulose CFC Assays to Detect Hematotoxicity for Compounds Affecting Cell Differentiation
In contrast to HALO®-Tox HT, which quantitatively measures functional viability and cell proliferation, or the lack of it due to cytotoxicity, the methylcellulose colony-forming cell (CFC) assay relies on the differentiation and maturation of cells, grown in colonies, in order to identify the cell that gave rise to the colony. Although the CFC assay has been used for many decades, it is not a robust, reliable or repeatable assay. Nevertheless, it is often requested and provided for toxicity studies.
HemoGenix® has developed three formats for the CFC assay all of which are available for contract services.
- ColonyGro™: A traditional 35mm Petri dish format performed using a minimum of duplicate replicates and a total culture volume of 1mL/dish. Colony enumeration is manual and occurs after 12-14 days incubation.
- CAMEO™-4: A miniaturized version of ColonyGro™ performed in quadruplicate using 4 wells incorporated into a 35mm Petri dish. The total assay volume is 0.1mL/well and the incubation time is between 9 and 12 days. Colony enumeration is manual.
- CAMEO™-96: Originally the first 96-well methylcellulose hematotoxicity assay developed in 2002, CAMEO™-96 is both a proliferation and differentiation assay. Cultures for CFC are setup in 96-well plates and colonies are counted between 4 to 7 days. The proliferation within the colonies is then quantitatively measured using the same standardized and validated ATP bioluminescence readout used for HALO®-Tox HT. The number of colonies correlates directly with the intracellular concentration of ATP. This correlation allows the CFC part of the assay to be standardized. Thus, CAMEO™-96 is the only true standardized CFC assay available.
All CFU assays are available either as (1) A Complete Service, Full Report, or (2) A Rapid Toxicity Study.
Cell Type
|
CFC Population |
Growth Factor Cocktail |
Pathways |
Predictive Toxicity |
CFC Assays Available |
|
|---|---|---|---|---|---|
| Primitive hematopoietic stem cell: Granulocyte, Erythroid, Macrophage, Megakaryocyte |
CFC-GEMM 1 |
EPO, GM-CFC, IL-3, IL-6, SCF, TPO, Flt3-L | Hematopoietic only |
Anemia Neutropenia Thrombo-cytopenia |
ColonyGro™ CAMEO™-4 CAMEO™-96 |
| Primitive erythropoietic progenitor cell: Stem cells induced into the erythropoietic lineage |
BFU-E 1 |
EPO, IL-3, SCF | Erythropoietic lineage | Anemia | ColonyGro™ CAMEO™-4 CAMEO™-96 |
| Primitive erythropoietic progenitor cell: Primitive erythropoietic progenitors already in compartment |
BFU-E 2 |
EPO alone | Erythropoietic lineage | Anemia | ColonyGro™ CAMEO™-4 CAMEO™-96 |
| Primitive granulocyte-macrophage (GM) progenitor: Stem cells induced into the GM lineage |
GM-CFC 1 |
GM-CSF, IL-3, SCF (similar to H4534) |
Granulocyte /macrophage lineage |
Neutropenia | ColonyGro™ CAMEO™-4 CAMEO™-96 |
| Primitive granulocyte-macrophage progenitor: Primitive GM progenitors already in compartment |
GM-CFC 2 |
GM-CSF, G-CSF, IL-3, SCF |
Granulocyte /macrophage lineage |
Neutropenia | ColonyGro™ CAMEO™-4 CAMEO™-96 |
| Primitive megakaryocyte progenitor: Stem cells induced into the megakaryopoietic lineage |
Mk-CFC 1 |
TPO, IL-3, SCF |
Megakaryopoietic lineage | Thrombocytopenia | ColonyGro™ CAMEO™-4 CAMEO™-96 |
| Erythropoietic precursor cell: Colony-Forming Unit - Erythroid | CFU-E | EPO alone | Erythroid lineage | Anemia | ColonyGro™ CAMEO™-4 |
| Granulocyte precursor cell: Granulocyte - Colony-Forming Cell | G-CFC | G-CSF | Granulocyte lineage | Neutropenia | ColonyGro™ CAMEO™-4 |
|
Macrophage precursor cell: Macrophage - Colony-Forming Cell < |
