In Vitro Renal Toxicity Testing
HemoGenix® is a Compliant Contract Research Service Supplier through Scientist.com
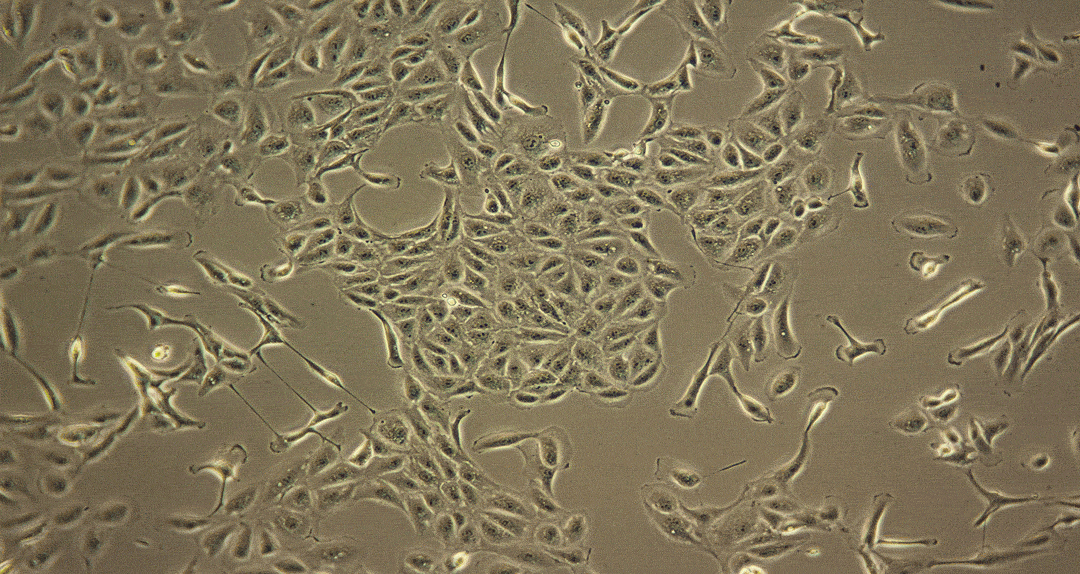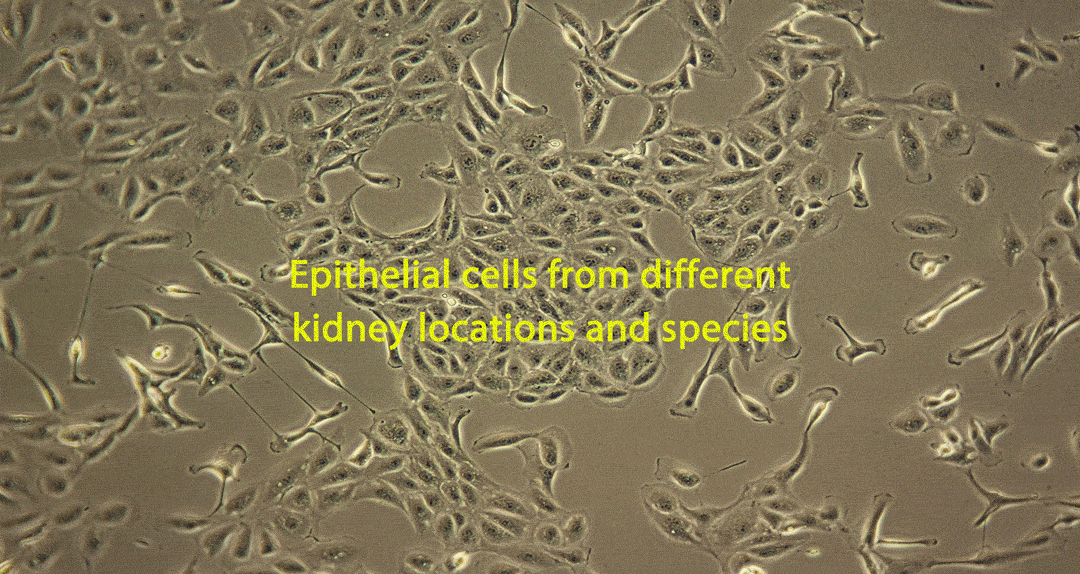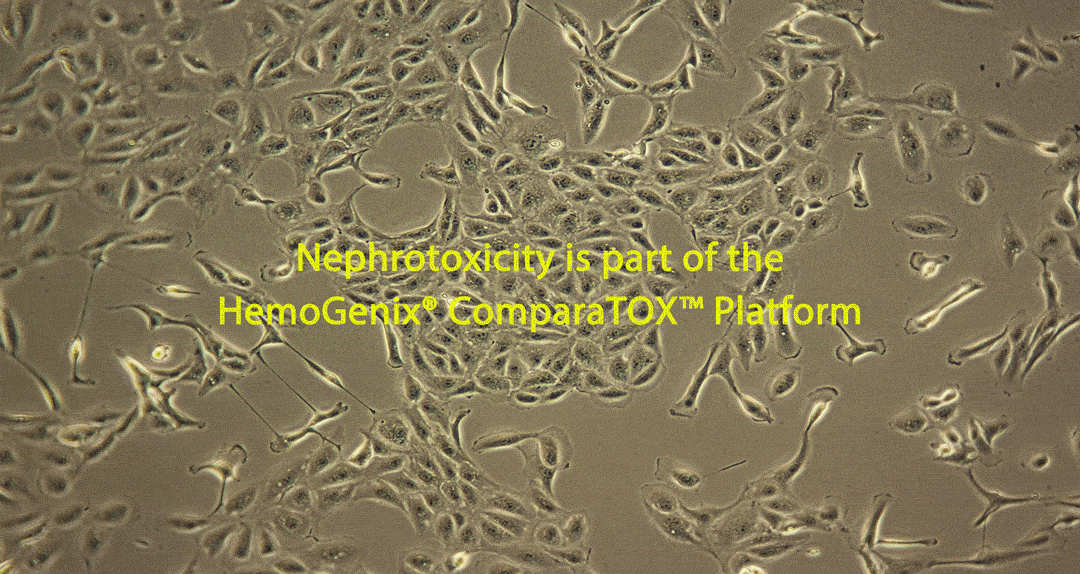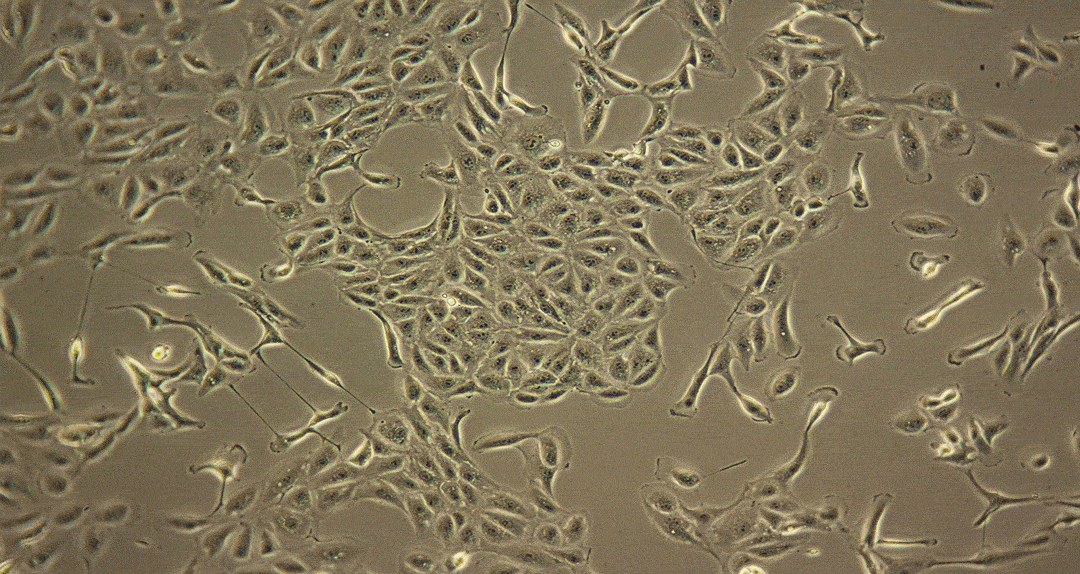
Damage caused by drug-induced kidney toxicity, also called renal or nephrotoxicity, is indicated when the kidney cannot detoxify or perform its excretion function. Many substances can lead to end-stage kidney disease. Renal toxicity can occur on different parts of the nephron, namely the glomerulus and proximal and distal tubules. There are biomarkers for these different nephron segments. There are also many different ways to study renal toxicity by using isolated perfused kidneys, renal tissue slices, isolated nephrons and their segments and even 3D cultures to try and maintain the complex kidney integrity.
Perhaps a more straightforward direction to testing and understanding renal toxicity, is to use RenalGlo™-Tox HT, an in vitro toxicity assay platform that can produce more rapid, predictive results of isolated primary cells.
For more information on renal toxicity testing, please contact HemoGenix® at contractresearch@hemogenix.com or call (719) 264-6250.
Types of Toxicity Studies Available
The majority of studies performed by HemoGenix® incorporate a Complete Service, Full Report that can be used for an IND application. More recently, Sponsors have requested a more streamlined study and studies involving high-throughput screening. For this reason, HemoGenix® now provides 3 types of study format.
- Complete Service, Full Report Study: Fully customized study that includes the Study Plan, Draft Text and Final Text Report with QA audit.
- Rapid Toxicity Study: A customized study that includes the Study Plan and full protocol, raw results and graphical data in a single Excel Workbook. No formal text report and no QA audit is performed. This type of study is designed for early drug development. No interpretation or conclusions are provided.
- High-Throughput Screening Study (presently only available for human studies): A screening study is designed for high-throughput screening of compounds during ADME/Tox screening in multiples of 5 on specific cell populations to provide the most important ranking information. The full protocol, raw results and graphical data are provided in an Excel Workbook. No interpretation or conclusions are provided. This type of Screening Study is part of the ComparaTOX™ HT Platform.
RenalGlo™-Tox HT for Renal Toxicity Testing
Although primary kidney cell types can be obtained from several species for renal toxicity testing, renal epithelial cells are usually used for human in vitro toxicity testing. Many of these cell types have been applied for use in RenalGlo™-Tox HT.
RenalGlo™-Tox HT is one of many in vitro toxicity testing platforms developed by HemoGenix®, starting with HALO®-Tox HT in 2002. Together with CardioGlo™-Tox HT and SkinGlo™-Tox HT, RenalGlo™-Tox HT is another ATP bioluminescence-based in vitro toxicity assay platform that can be used in parallel to produce a unique comparative toxicity testing platform called the ComparaTox™.
RenalGlo™-Tox HT for In Vitro Drug-Induced Renal Toxicity
- Many renal cell types produce high concentrations of intracellular ATP (iATP) due to their high metabolic activity. Isolated primary epithelial cells also demonstrate proliferation ability, which correlates with iATP concentration. Changes in iATP concentration directly correlate with cellular and mitochondrial integrity and cytotoxicity.
- Renal cells from various sources and species can be used with RenalGlo™-Tox HT.
- RenalGlo™-Tox HT incorporates a validated ATP bioluminescence readout to measure nephrotoxicity.
- RenalGlo™-Tox HT is a calibrated and standardized assay platform that allows results to be directly compared between different drugs, renal cell sources and species over time.
- High-throughput capability using 96- or 384-well plate formats allows ADME-Tox drug or compound screening, thereby significantly reducing unexpected results during pre-clinical testing.
- RenalGlo™-Tox HT can be multiplexed with assay readouts.
- Incorporates the most sensitive ATP bioluminescence readout available.
- Study Turnaround time: Usually within 7 days.
- Validated assay readout according to FDA Bioanalytical Method Guidelines.
- Supports the 3Rs (Reduction, Refinement, Replacement) assay platform for animal testing.
Sources of Renal Cells for RenalGlo™-Tox HT
Renal epithelial cells derived from:
- Medulla
- Glomerulus
- Distal tubule
- Proximal tubule
- Cortex
In addition, RenalGlo™-Tox HT can also be used with virtually any kidney cell lines.
Examples of Nephrotoxicity to Human Proximal Tubule Cells
Using Google Chrome browser? Right click on image icon. Then click "Open Image in New Tab"
Species Available for Use with RenalGlo™-Tox HT
RenalGlo™-Tox HT can be used with human kidney cells as well as kidney cells from other species.
Please contact HemoGenix® for more information.
Multiplexing Capabilities with RenalGlo™-Tox HT
- Membrane integrity: LDH or PI dye exclusion
- Apoptosis: Biochemical caspase detection
- Mitochondrial dysfunction: Mitochondrial ToxGlo™
- Glutathione Assay (GSH): Oxidative stress
- OxyFLOW™: Oxidative DNA damage
Please also view the Mechanism of Action page.
Comparing and Ranking Drug-Induced Nephrotoxicity with Toxicity to Other Biological Systems
This unique in vitro toxicity testing platform is called ComparaTOX™. It is based on the fact that other HemoGenix® toxicity assays use exactly the same readout, namely a standardized and validated ATP bioluminescence signal detection system. For example, HALO®-Tox HT, ImmunoGlo™-Tox HT, MSCGlo™-Tox HT, HepatoGlo™-Tox HT, NeuroGlo™-Tox HT and CardioGlo™-Tox HT. Although each assay platform has been designed for a different biological stystem, they all use the same readout. When combined together into the ComparaTOX™Platform, the results from each assay platform can be directly compared with each other. This allows the response to drugs and other agents to be ranked according to:
- Drug toxicity
- Cell type
- Species
Assay Kits for In-House Renal Testing
You can perform in vitro renal toxicity testing in-house using assay kits sold by Preferred Cell Systems™. Please click on the links below to take you to the assay kit page on the Preferred Cell Systems website.
